The drug candidate LIB-01
"Our goal is to develop a next-generation erectile dysfunction drug with long-lasting effect and that helps many more. Our results clearly demonstrate that we are moving towards this goal, which would make a significant difference for affected men and couples worldwide."
– CEO Elin Trampe
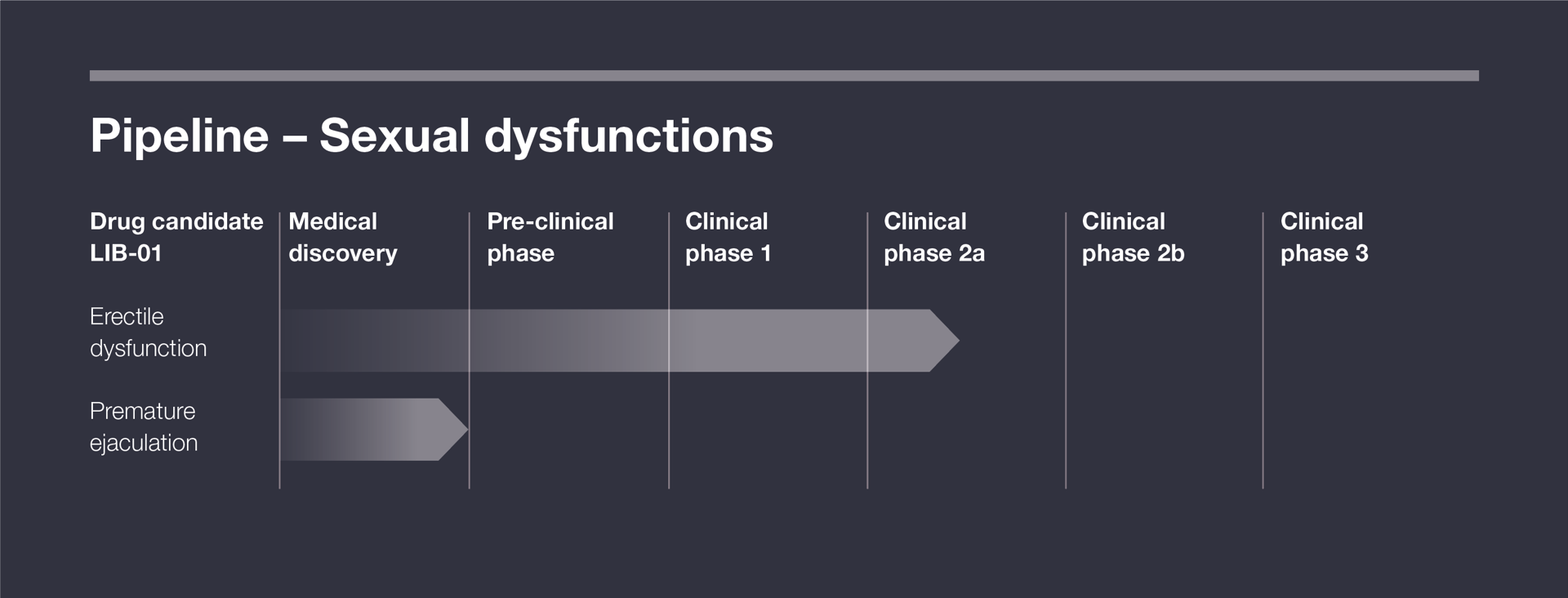
In 2023, the preclinical development program was completed, and the first human clinical study was initiated the same year. It demonstrated that LIB-01 has a very strong safety profile, which was the study’s primary objective. Additionally, a clear efficacy signal was observed, with participants reporting improved erectile function lasting four weeks—an entirely unique finding in the field.
In 2024, a Phase 2a study was initiated to establish statistical significance for LIB-01’s effect on erectile function. The clinical part of the study is expected to continue until mid-2025, followed by statistical analysis before results can be reported.
The development program also includes process development and manufacturing scale-up to ensure access to the active substance, which, together with a suitable formulation, constitutes the final product now being tested in clinical trials.
New research findings within the LIB-01 development program indicate that the substance may also have an impact on factors related to metabolic diseases, including conditions such as obesity and diabetes. A preclinical study is now underway in parallel with the development of the potency drug candidate.